Cipla Usfda Warning Letter 2025. We wish to inform you that on november 18, 2025, the company has received a warning letter dated november 17, 2025 from [the] united states food and. 2 min read 24 nov 2025, 04:35 pm ist trade now.
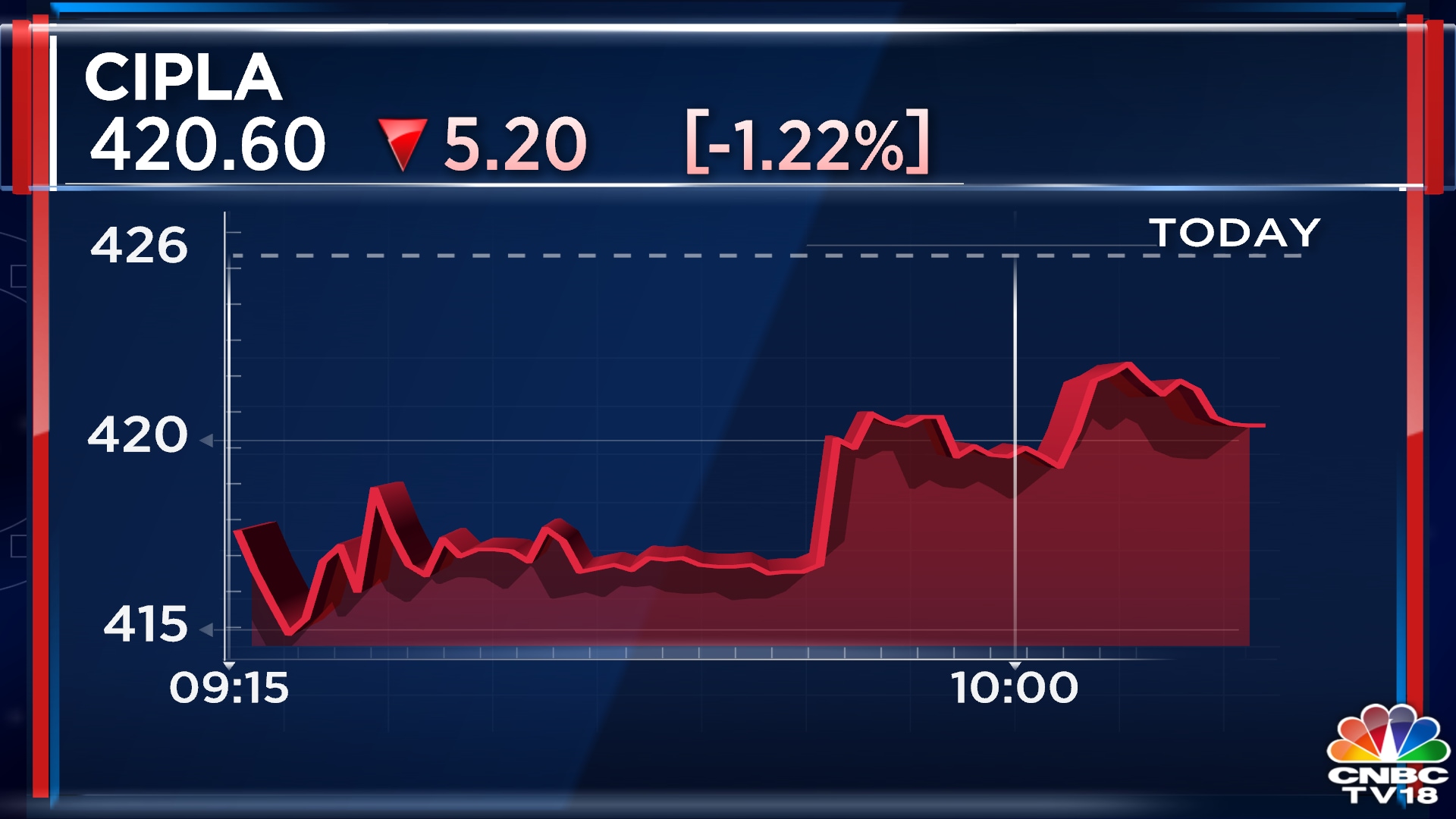
Shares of drug firm cipla tumbled nearly 6 per cent on wednesday after the us health regulator issued a warning letter for its goa manufacturing facility. Drug firm cipla ltd on wednesday said that the us health regulator has issued a warning letter to the company for its.
This warning letter summarizes significant violations of current good manufacturing practice (cgmp) regulations for finished pharmaceuticals.

FDA Warning Letters und Formular 483, The warning letter issued to cipla will further delay the launch timelines of the company’s advair generic in the us. Last updated on december 30, 2025 by the health master.

Cipla shares fall after receiving warning letter from USFDA Angel One, Drug firm cipla ltd on wednesday said that the us health regulator has issued a warning letter to the company for its. The usfda warning letter outlined violations related to methods or controls at the facility that did not comply with prescribed cgmp regulations.

Warning Letter Template Format, Sample & Example in PDF & Word, Drug firm cipla ltd said that the us health regulator has issued a warning letter to the company for its. Cipla fda form 483 plant inspections.

FDA issues Form 483 to Cipla; warning letters to Torrent, Glenmark, Food and drug administration (us fda) for the routine current good manufacturing. Updated on feb 27, 2025 at 07:10 am ist.

USFDA issues cautioning letter to Cipla for Goa plant Laboratory, The company further said that. The usfda warning letter, dated november 17, 2025 , follows a routine cgmp inspection at the pithampur manufacturing facility, undertaken between february.

Why Does USFDA Issues Warning Letter?, Shares of pharma major cipla fell as much as 8% in intraday trade on thursday following a warning letter by the usfda for various manufacturing lapses at its. The company further said that.
Cipla Receives Warning Letter From Usfda, Shares Fall, Updated on feb 27, 2025 at 07:10 am ist. The us health regulator has pulled.

USFDA inspection at Cipla manufacturing facility in Goa, warning letter, Cipla receives usfda warning letter following inspection at pithampur site. Last updated on december 30, 2025 by the health master.

How to search warning letters on USFDA site USFDA साइट मे वार्निंग लैटर, The warning letter issued to cipla will further delay the launch timelines of the company’s advair generic in the us. Cipla receives usfda warning letter following inspection at pithampur site.

USFDA issues warning letter to Cipla for Goa manufacturing facility, ET, Last updated on december 30, 2025 by the health master. Posted date letter issue date company name issuing office subject response letter closeout letter excerpt;
The usfda warning letter outlined violations related to methods or controls at the facility that did not comply with prescribed cgmp regulations.
This warning letter summarizes significant violations of current good manufacturing practice (cgmp) regulations for finished pharmaceuticals.